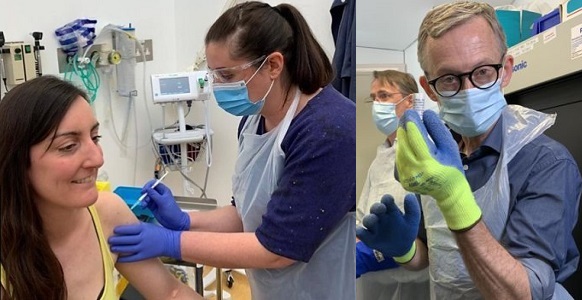
The first human trial in Europe of a coronavirus vaccine has begun in Oxford.
Two volunteers were injected out of more than 800 people recruited for the study.
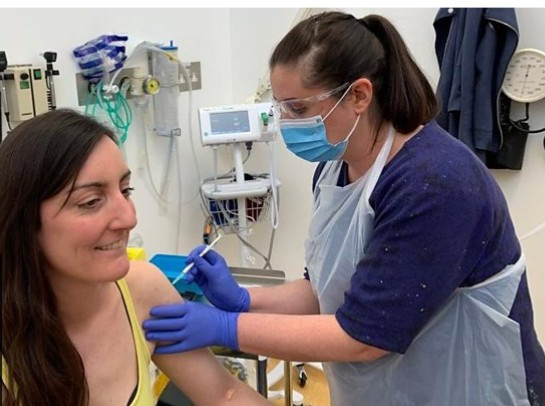
Elisa Granato, pictured above, is a scientist and was the first volunteer to be injected. Elisa said she volunteered because she wanted to try to support the scientific process.
Half of those who volunteered will receive the Covid-19 vaccine, and half a control vaccine which protects against meningitis but not coronavirus.
The design of the trial means volunteers will not know which vaccine they are getting, though doctors will.
The vaccine was developed in under three months by a team at Oxford University.
Sarah Gilbert, professor of vaccinology at the Jenner Institute, led the pre-clinical research.
“Personally I have a high degree of confidence in this vaccine,” she said.
“Of course, we have to test it and get data from humans. We have to demonstrate it actually works and stops people getting infected with coronavirus before using the vaccine in the wider population,” she added.
The Coronavirus vaccine is made from a weakened version of a common cold virus (known as an adenovirus) from chimpanzees that has been modified so it cannot grow in humans, according to BBC.
The Oxford team has already developed a vaccine against Mers, another type of coronavirus, using the same approach – and that had promising results in clinical trials.
The only way the Oxford team will know if the Covid-19 vaccine works is by comparing the number of people who get infected with coronavirus in the coming months from the two arms of the trial.

That could be a problem if cases fall rapidly in the UK, because there may not be enough data.
Prof Andrew Pollard, director of the Oxford Vaccine Group, who is leading the trial, said: “We’re chasing the end of this current epidemic wave. If we don’t catch that, we won’t be able to tell whether the vaccine works in the next few months. But we do expect that there will be more cases in the future because this virus hasn’t gone away.”
The vaccine researchers are prioritising the recruitment of local healthcare workers into the trial as they are more likely than others to be exposed to the virus.
A larger trial, of about 5,000 volunteers, will start in the coming months and will have no age limit.
The Oxford team is also considering a vaccine trial in Africa, possibly in Kenya, where the rates of transmission are growing from a lower base.
The trial volunteers will be carefully monitored in the coming months. They have been told that some may get a sore arm, headaches or fevers in the first couple of days after vaccination.
They are also told there is a theoretical risk that the virus could induce a serious reaction to coronavirus, which arose in some early Sars animal vaccine studies.
But the Oxford team says its data suggests the risk of the vaccine producing an enhanced disease is minimal.
Another team at Imperial College London hopes to begin human trials of its coronavirus vaccine in June.
The Oxford and Imperial teams have received more than £40m of government funding.